The boater's guide to corrosion
This guide is intended to give you a fundamental understanding of what corrosion is, what causes and affect it, and help you determine which anode material is right for your boat.
Of all the complicated subjects a boat owner needs to know, corrosion is probably the most confusing. Corrosion can cause implications in both boat maintenance and boat safety. Certain kinds of corrosion can even devalue your investment in a matter of months or even days.
What is corrosion?
Corrosion is an electrochemical process of deterioration of metal components when exposed to an aqueous environment, such as water. This occurs both underwater and in the atmosphere.
Deterioration is the process of which the metal is changing into its oxide form. Steel, for example, will degrade (oxidize) back to its natural stable state, namely rust.
Corrosion mechanism
The metal atoms at the surface give up electrons and turn into positively charged ions, which dissolve into the water or electrolyte (a liquid that can conduct electricity). Electrons flow through the metal from the corrosion area to other areas close by, where they form negative ions in the water. The positive ions flow through the water and combine with the negative ions flowing in the opposite direction.
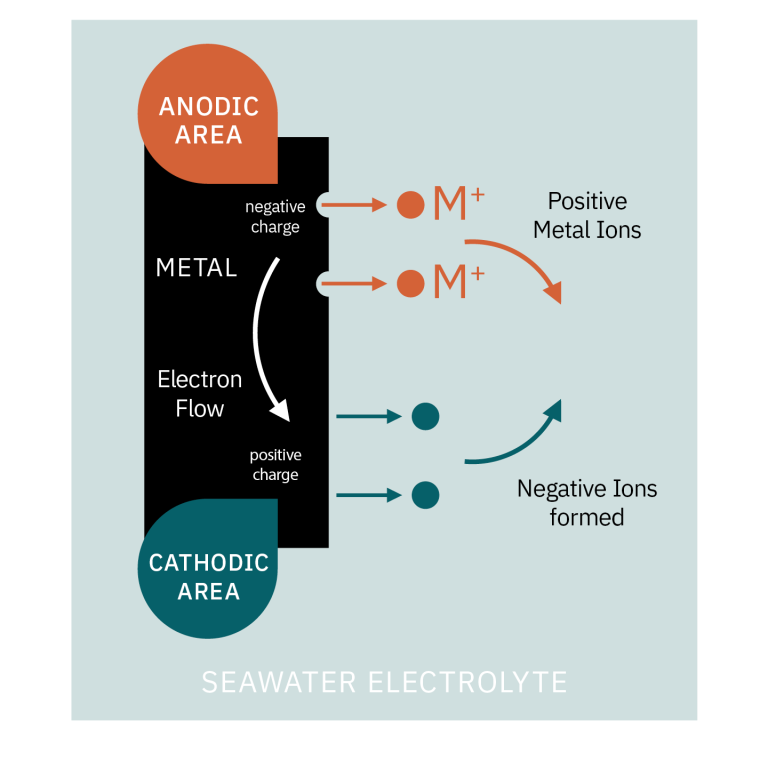
So, you can see that an electric current is set up between localized areas on the metal surface, resulting in the metal loss (corrosion) at the anodic areas. Only electrons are given up at the cathodic areas, so no metal is lost in these areas. They are, in fact, protected.
Galvanic corrosion
When two different metals (copper and steel in the example) are in contact, electrons will flow from the more negatively charged metal (anode) to the more positive metal (cathode). The voltage generated between copper and steel would be 0.3 volts. The circuit is completed by the loss of positively charged ions, from the anode into the electrolyte, and the negatively charged ions at the cathode.
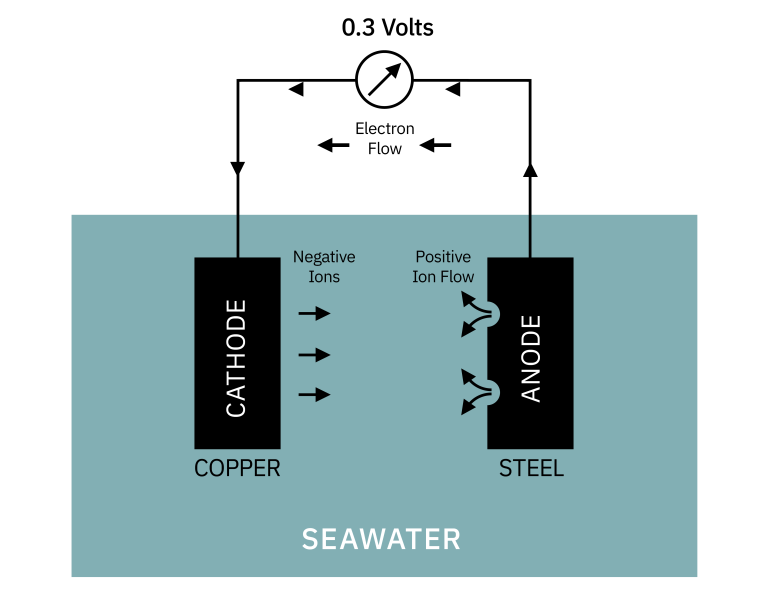
This release of small particles (ions) into the water is much more rapid than with one metal alone and is limited to the steel's corrosion. The cathode material (copper) is protected.
Sacrificial anode
If you want to protect both types of metal, you must add a third more active metal. The most common metal is zinc, although magnesium and aluminum are also used. This active metal becomes the anode for both metals.
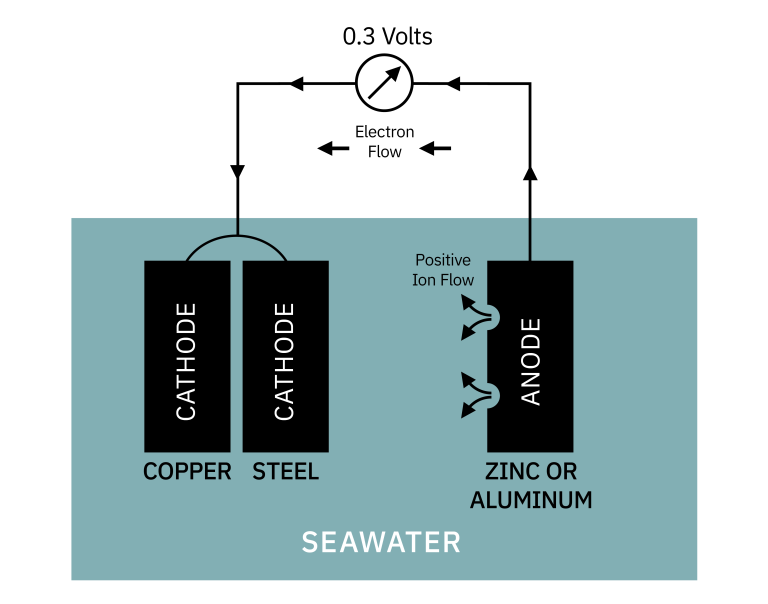
The zinc or aluminum sacrifices itself to protect the other two metals, hence the term sacrificial anode.
Why do some metals corrode more than others?
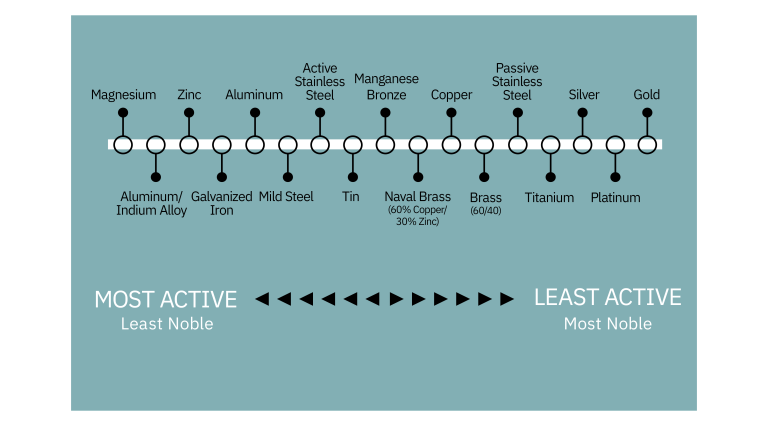
All metals tend to be oxidized (corrode), some more easily than others. The relative rate can be plotted on the galvanic series:
What factors affect corrosion?
Be aware that some of these factors can vary microscopically at the surface of the metal.
- Conductivity of electrolyte - seawater is a good conductor and freshwater is a bad conductor, so corrosion is worse in seawater.
- Amount of oxygen - generally, corrosion rates increase in proportion to the amount of oxygen in the water. However, cracks and crevices, which are areas starved of oxygen, become anodic and corrode also.
- Presence of pollutants - increases corrosion.
- Flow rate - will increase corrosion rates. Pitting in stainless steel is reduced, however.
- Temperature - higher temperature increases corrosion rates, approximately doubling for every 10°C/18°F
- Stress - metal under tensile stress (stretched) in combination with corrosion can suffer sudden failure due to stress cracking.
- Presence of bio-organisms - there are various types of microorganisms that can contribute to corrosion, either by removing protection or causing a corrosive environment.
Area of weight and anodes
The surface area of the sacrificial anodes determines how much protection (amperage) you get. The weight determines how long they will last. Different anodes have different capacities measured in amp-hours per pound.
Cathode to anode ratio
The ratio of the cathodic (protected) area to the anodic (corroding) surface is critical in galvanic corrosion. The smaller the area where the anode is giving up material, the faster it will take place. Ideally, the anodic area should be much bigger than the cathodic area. This ratio can be improved by painting the cathodic surface.
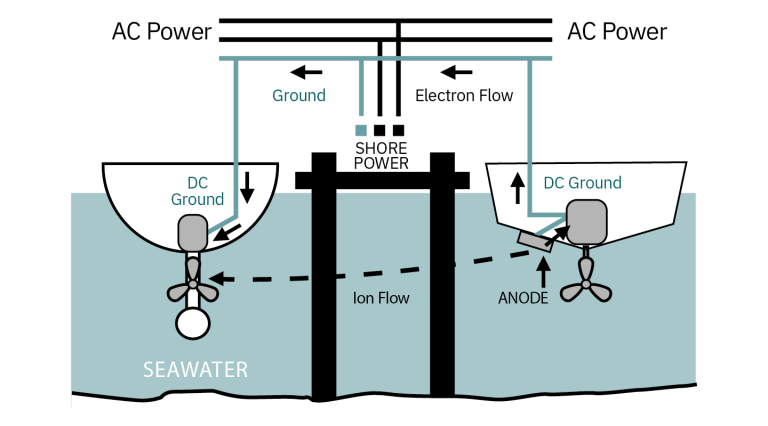
Galvanic corrosion via the ground wire
Connecting into shore power connects your ground to the neighboring boats. If suitable anodes do not protect these boats, you will be the one protect them – causing rapid wearing of your anodes. See the diagram below:
Galvanic isolator
A galvanic isolator is a device that is installed in the green ground wire to block direct galvanic currents but still allow AC to pass. Beware: Make sure your galvanic isolator is rated for the power you use, e.g., 30A or 50A. Poor quality galvanic isolators have been known to start fires, so it's a good idea to get one, which is ABYC recognized or UL listed to ensure that it has been properly tested.
Stray current (electrolytic) corrosion
This corrosion is caused by an external current flowing from a battery or other DC source. This current flows out of the metal into the water and causes loss of material or corrosion in the process. Common causes include a bare wire in the bilge on incorrectly wired or installed equipment.
Impressed current system
Instead of using a sacrificial anode to generate a protective voltage, a DC power source can be used. The principle is the same, but the current is monitored and adjusted by the system. A non-corroding material is used for the anode.
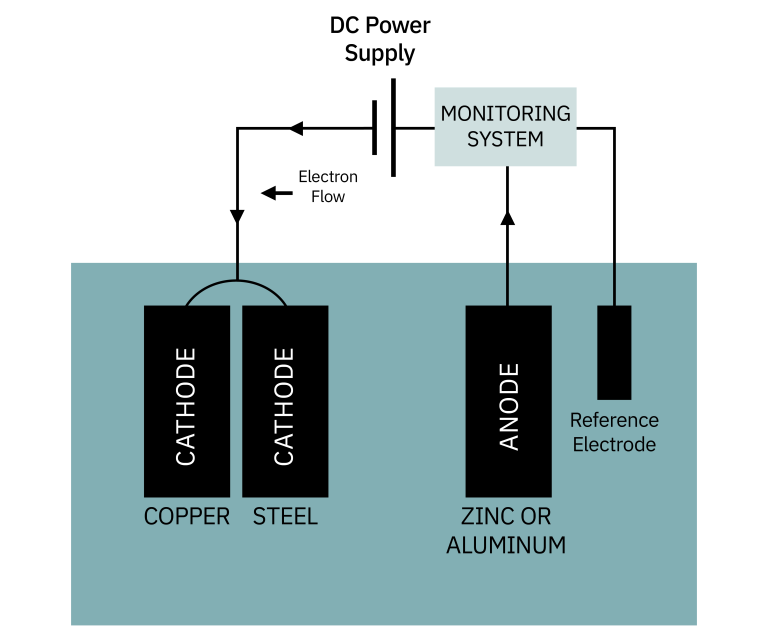
The advantage of an impressed current system is that it can develop higher voltages than a sacrificial anode. The disadvantage is that it can "over-protect." Impressed current systems are used on all types of boats and sterndrives.
Bonding
All electrical equipment and underwater metal fittings should be connected to the same ground point (connected to the battery negative terminal). This ensures that all components are at the same voltage, preventing any stray currents occurring.
Sacrificial anode materials
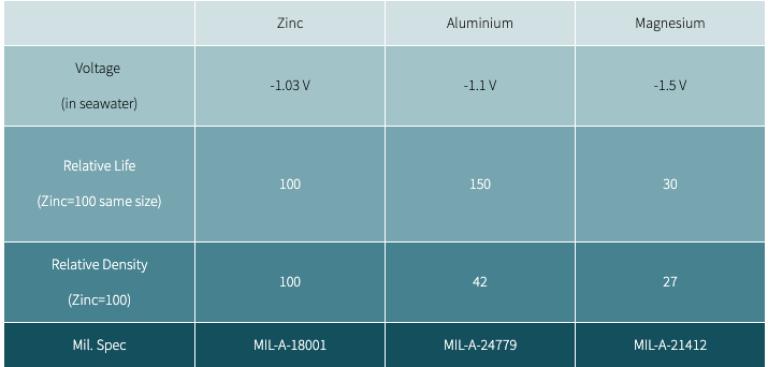
Zinc anodes
Zinc is the most common material used. Zinc anodes are not very useful in freshwater and can stop working after only a few months if not made to millimeter specifications. It is a good policy to change them regularly, even if they look ok. Remember, if an anode doesn't wear away, it is not working!
Aluminum anodes
The aluminum alloy used in anodes is very different from ordinary aluminum. It includes about 5 percent zinc and a trace of Indium, which prevents an oxide layer's build-up. Aluminum anode alloy provides more protection and lasts longer than zinc (see chart). It will continue to work in freshwater and is safe for use in saltwater. Aluminum is the only anode that is safe for all applications.
Magnesium anodes
Magnesium is the most active metal on the galvanic scale. It can be used in freshwater, but care must be exercised. Magnesium can over-protect aluminum hulls or outdrives in salt or brackish water or even polluted freshwater, causing the paint to be lifted with resulting corrosion. Even a few hours of immersion can cause severe damage.
Which anode material is right for your boat?
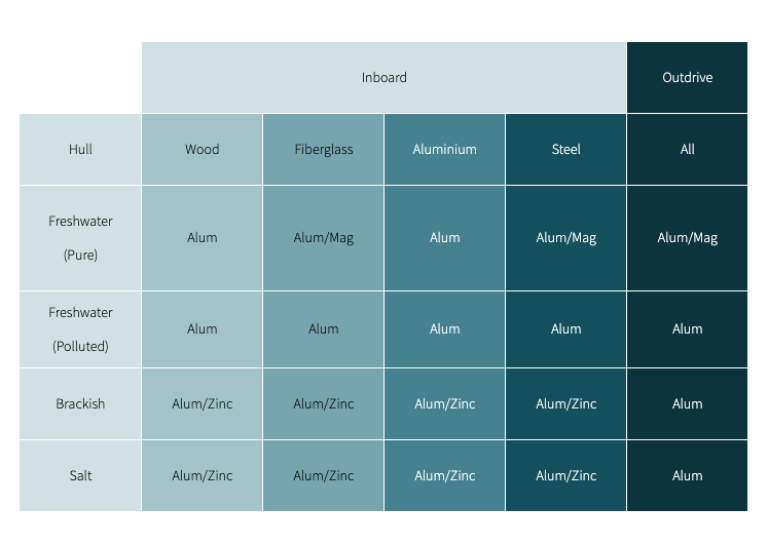
Anode do's and don'ts
Do
- Change your anodes when they are 50 percent corroded. a 'wear indicator' anode will help tell you when to change.
- Make sure they make good electrical contact - remove paint and clean the mounting surface.
- Protect trim tabs individually (do not bond). Although they are usually made from stainless steel, they can still corrode and need sacrificial anodes.
- On sterndrives, be sure to use new fasteners (usually supplied with the anode) - even stainless bolts fail due to corrosion.
- Keep a sterndrive immersed in the water so that the anodes can work.
Don't
- Do not paint anodes. They will not work!
- Do not mix anode types - aluminum anodes will try to protect zinc anodes on the same bonding circuit.
- Do not use zinc anodes on aluminum outdrives - they will not provide the proper protection.
- Do not use magnesium anodes on outdrives in salt or brackish water as they will 'overprotect' the aluminum.
Common marine materials facts
Aluminum
Aluminum is an excellent material for marine use. Aluminum is a light, strong metal that is easy to work with. It has excellent corrosion resistance due to its ability to form a protective oxide surface film rapidly. Unprotected, it may become pitted or covered with a gritty white powder, but these are usually superficial and not harmful. Anodizing eliminates this.
However, it is very active on the galvanic series (-.76 to -1.00 volts), which makes it prone to galvanic corrosion when in contact with more noble metals. Bronze, brass, or monel fittings should be avoided or insulated to prevent galvanic action. Stainless steel (316) fasteners are recommended. Aluminum can be overprotected by too much voltage from magnesium anodes in salt, brackish or polluted freshwater.
Brass
Brass is an alloy of copper and zinc. Generally not recommended for exposed use. Brass suffers from dezincification, which is the galvanic corrosion of the zinc from the alloy, leaving a spongy brittle component. Be aware that manganese bronze is brass, not a true bronze, and needs galvanic protection if used underwater.
Bronze
Bronze alloys of copper with little or no zinc. Authentic bronzes are robust and extremely resistant to corrosion, both in the atmosphere and immersed. Bronzes may contain tin, aluminum, nickel, or phosphorus, but the best and most widely used is silicon bronze. Widely used in fittings and fasteners.
Stainless steel
Stainless steel is a widely used strong corrosion-resistant material. Stainless owes its corrosion resistance to its chromium content, which forms an oxide film resistant to attach (material is then referred to as passive). Nickel improves welding properties. 18 percent chromium and 8 percent nickel is the minimum grade, 304. Even better is 316 grade, which has molybdenum, which enhances corrosion resistance.
If stainless is starved of oxygen (e.g., under seals or barnacles), it loses its protective oxide film and becomes active. It will then corrode readily. This can also occur in microscopic crevises resulting in almost invisible corrosion, which can cause sudden failure. Suitable for deck fittings, it is not recommended for use underwater, except when galvanically protected as, for example, a fastener in an aluminum outdrive.
Wood hulls
Wood hulls are very prone to deterioration due to various types of wood rot and corrosion caused by metal fittings and fastenings. Silicon bronze fasteners are recommended. Do not use stainless below the waterline.
Fiberglass/composite hulls
With fiberglass/composite hulls, silicone bronze fasteners are recommended below the waterline. Warning: Carbon (graphite) fibers are electrically conductive and can cause galvanic corrosion between metal components in the structure.
Useful links
- The truth about aluminum anodes - by Martin Wigg and Paul Fleury (pdf)
- Basics of corrosion - by Performance Metals (pdf)
- Sacrificial anodes FAQ - by Performance Metals (pdf)
Reference: Performance Metals

